Using living cells to understand how neurons speak to one another
Our brains are complex organs with intricate networks of neurons. Day in and day out, neurons are consistently active within various neural circuits in our brains. Though research on the function of the brain and its neurons is rapidly advancing, many areas remain unexplored. For instance, the mechanisms underlying communication between neurons is yet to be elucidated.
Associate Professor Saitoh is engaged in research to “see” the live movements of molecules within neurons. “Neurons communicate with surrounding cells using a molecular ‘language,’ similar to how people use words to communicate with each other,” he says. “My research focuses on ‘how cells speak.’ I observe and analyze the molecular dynamics that take place within living cells (i.e., live cell imaging of neurons) to understand how cells use their words, speak to one another, and have their intentions communicated.”
In the life sciences, while molecular biology and biochemistry deal with molecules from inactive cells that have been purified or fixed to investigate the function and distribution of specific molecules, physiology deals only with living and functioning cells. Dr. Saitoh, who specializes in research on the functional analysis of the central nervous system, believes that understanding the movement of molecules within neurons will help elucidate the mechanisms that are involved in the development of diseases and disorders of the neural circuitry.
The successful development of a fluorescent sensor for the visualization of cAMP, an important molecule for the expression of cellular functions
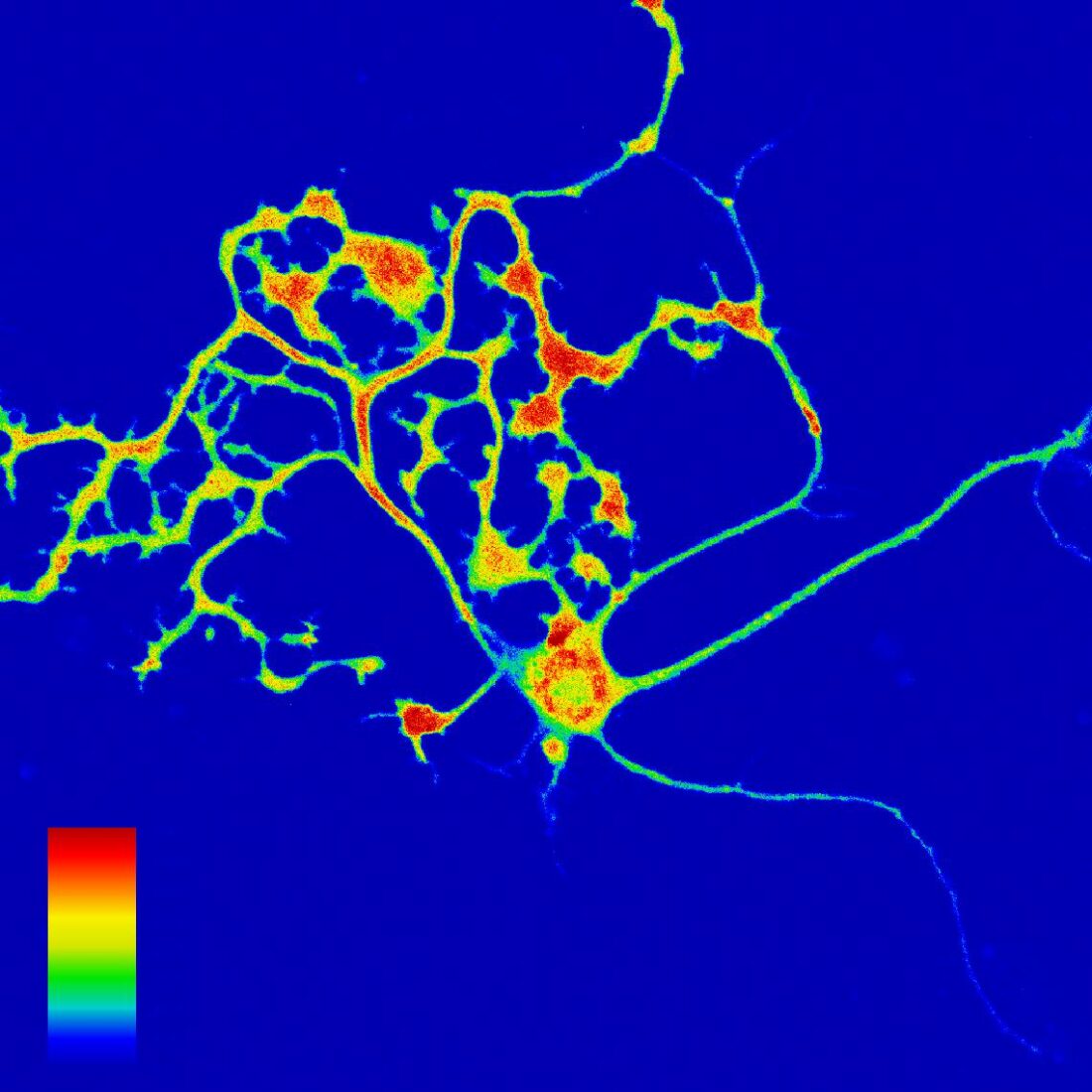
In particular, Dr. Saitoh is passionate about exploring the real-time imaging of cAMP (cyclic adenosine monophosphate)—a molecule involved in learning and memory formation. It is a second messenger that activates intracellular signal transduction systems. He observes, in real-time, the rise and fall of cAMP concentration levels in the neurons, and tracks the regions in which these fluctuations occur, the degree of fluctuations, and the timing at which they occur. “To observe the movement of colorless and transparent molecules in living cells through optical microscopes, it is necessary to apply fluorescence to specific molecules. Since cAMP itself does not fluoresce, we attached a fluorescent protein that can bind to cAMP( Figure 1 ).” Based on the intensity of the fluorescence of the fused protein, Dr. Saitoh succeeded in visualizing the concentration of cAMP in neurons ( Figure 2 ). This “development of a cAMP-specific fluorescent sensor” is expected to lead to new neurofunction-related discoveries.
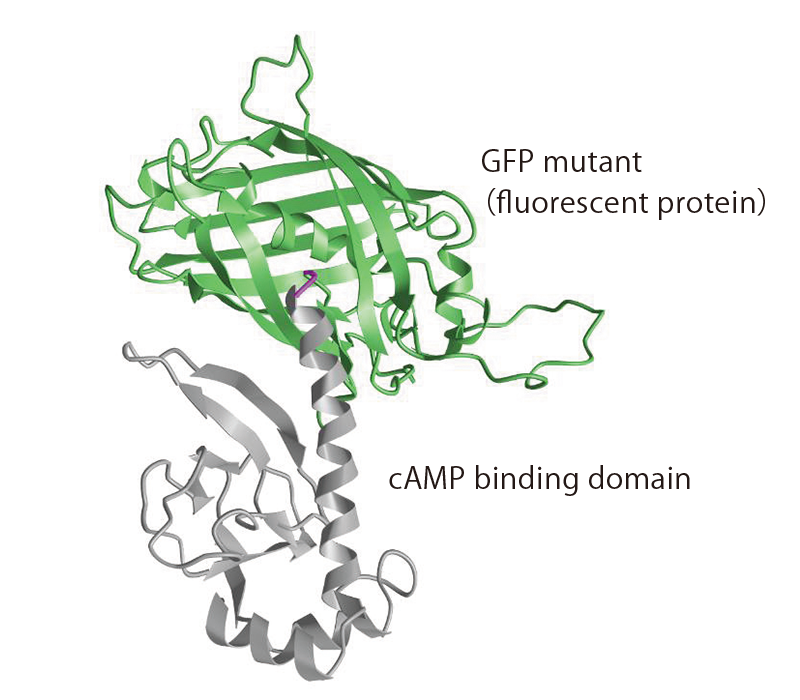
(Figure 1) Predicted steric structure of cAMP fluorescent indicator gCarvi
(Figure 2) Cultured Purkinje cells expressing gCarvi
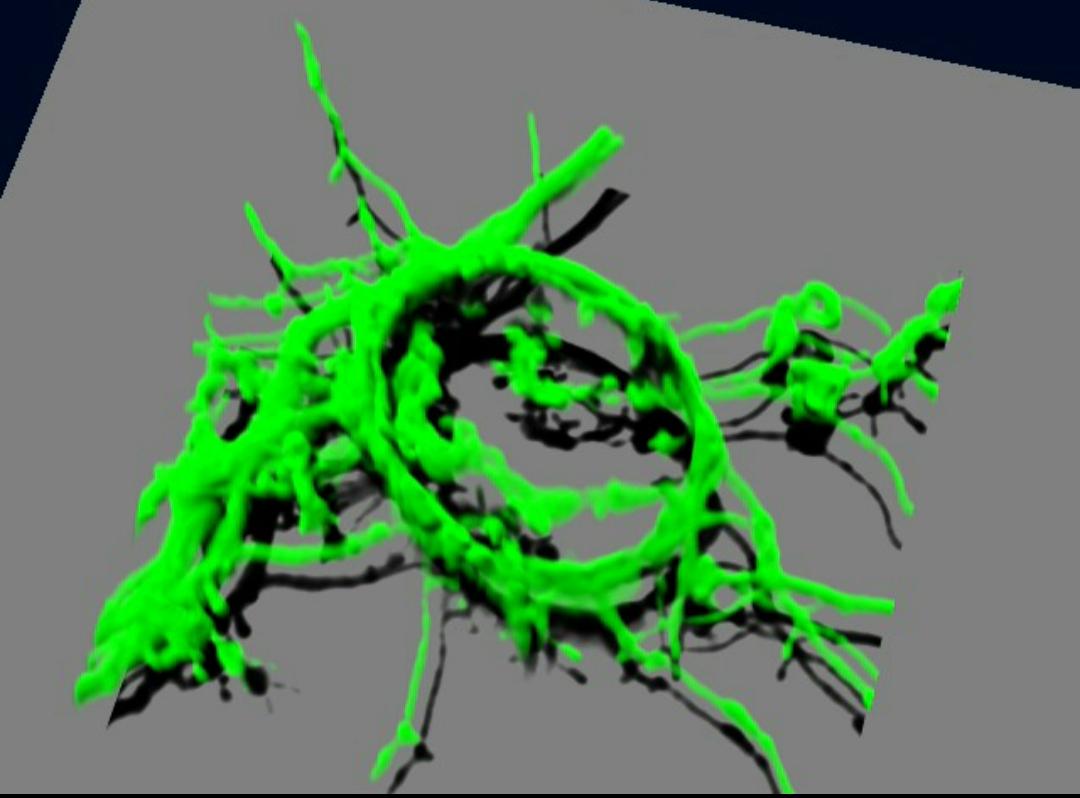
Furthermore, Dr. Saitoh has managed to culture the giant presynaptic terminals found in the brainstem: the calyx of Held synapse ( Figure 3 ), because almost all presynaptic terminals are structurally extremely small and difficult to observe under optical microscopes. Based on these successful experiments, he has unraveled new possibilities for the imaging of presynaptic terminals under optical microscopes.
According to Dr. Saitoh, these studies will contribute to our understanding of synaptic transmission regulatory systems, such as memory formation. “During the learning and memory formation process, long-term changes or long-term plasticity occurs as part of synaptic transmission. Among such long-term changes, those that last more than a day are grouped under late long-term plasticity—which is considered necessary for forming long-term memories. This late long-term plasticity will likely require an increase in cAMP concentration in the neurons’ nucleus. However, till date, no researcher has managed to observe this phenomenon. I believe my research will help uncover the reality of such a phenomenon,” muses Dr. Saitoh.
(Figure 3) Cultured Calyx expressing GFP
Dr. Saitoh also applied for a patent titled “cAMP Indicator and Its Manufacturing Method.” The findings of his research have reached a milestone, in that they are potentially applicable not only to neurons, but also to cells constituting other tissues. Furthermore, by analyzing the movement of intracellular cAMP via “GPCRs,” i.e., signal receptors on the cell surface, he hopes to contribute to research on drug discovery and target GPCRs related to various diseases.
Research on neural circuit homeostasis associated with Alzheimer’s disease: The way forward
Dr. Saitoh’s research is not only confined to cellular units, but also to neural circuits formed by neurons. He remarks, “While neural circuits are formed in cultures, the criterion to determine their formation is to maintain a stable spontaneous firing frequency. In cultured hippocampal neural circuits, the frequency is neither 10 Hz nor 0.1 Hz, but always at about 1 Hz. I believe that cAMP may be involved in the maintenance of homeostatic firing. However, the quantitative, temporal, and spatial cAMP dynamics are not yet known, and I would like to elucidate them using real-time cAMP imaging techniques.”
Dr. Saitoh adds that it is essential to understand the mechanism of neuronal circuit homeostasis to solve the mysteries of brain diseases. “Alzheimer’s disease, a brain disease, is caused due to the accumulation of amyloid-β, which leads to the death of neurons. Even if some neurons die or have defects in synaptic transmission, they should be able to readjust their circuits through the mechanism of homeostatic firing. By focusing on cAMP and elucidating the mechanism of enhancing neuronal homeostasis, I hope to advance my research to develop drugs that can prevent dementia,” he adds.
Dr. Saitoh has been steadily accumulating tangible results despite the many obstacles he has encountered along the way. He remarks that the joy of conducting research is closely linked to solving problems one by one and proving his hypotheses. “I am so fascinated by basic research that I cannot think of following any other path but that of a researcher,” he says, then adds with a smile, “I am making efforts to convey this fascination to my students. It would be a great honor for me if they contributed and enjoyed working in my field of research.” The enthusiasm and joy with which Dr. Saitoh hopes for his students’ success and the fruits of the development in his research field is truly inspiring.
