Observing brain activities with the aim of illuminating its functions
Neurophysiology is the scientific domain that investigates brain activities, including memory and learning. The aim is to determine brain-cell connection circuits (neurocircuits). Since “brain functions” is a broad term, this domain encompasses a wide range of fields, from basic psychology to cognitive neuroscience.
The development of observation methods is indispensable for the growth of this research domain. The key is to be able to capture the “real-time” state of the brain in more and more detail while it is actively working. Making those parts of the brain that are still largely unexplained visible is linked with the finding of novel facts. Dr. Masamizu, who specializes in neurophysiology, uses in vivo calcium imaging, a technique established around 2005, to explore and discover the true nature of the brain and its numerous mysteries. “We look at areas of the brain performing processing when an animal is performing a cognitive task, to determine how its brain cells (neurons) are acting. In in vivo calcium imaging, fluorescent calcium sensor proteins are used to monitor neural activity, which we then observe using a two-photon microscope and a macro zoom/multipoint laser scanning confocal microscope (Photo 1). This in vivo calcium imaging makes it easy to observe long-term activity changes in the same neurons. It is thus excellent for observing brain activity in animals used to perform experiments. Using this technique, we are clarifying neurocircuit networks involved in learning and memory, and more.”

Dr. Masamizu has been fascinated with the brain since his childhood. He selected a career as an academic researcher in order to learn and help unlock the principles of the brain. In his years as a graduate student, his research focused on the specialized field of neuroembryology, which studies the formation and development of the brain from the earliest stages. After he received his Ph.D., he moved to the field of neurophysiology. “I had a strong desire to know more and more about the brain, and thus studied brain development processes. However, as I pursued my research, I became aware of the need to know how the brain acts in order to acquire brain functions. This was the spark that led to my moving on to neurophysiology.”
Edging closer to new brain facts through step-by-step technological development
Dr. Masamizu uses in vivo calcium imaging to study rodents and primates. Their team was the first worldwide to determine that memory of learned movements is stored in a deep layer of the cerebral cortex. “The cortex has a six-layer structure. Prior research had observed layer 2/3 nearest the surface using in vivo calcium imaging. We made the special arrangement, which enabled the specific expression of fluorescent calcium sensors, used in in vivo calcium imaging, in deep layers. This enabled us to make observations of activity in deeper layers than before. As a movement task for a mouse, we devised an experiment where the mouse would receive water when pulling a lever for a fixed time. As result, we discovered that, in the late stage of learning, there was unique activity within layer 5 of the cerebral cortex motor area. We suggested that the neurons there might be responsible for motor-skills memory.”
Dr. Masamizu aims to reveal more about human brain functions. Using the marmoset—a primate that more closely resembles humans—to perform movement task experiments, he has also monitored neuronal activities in the marmoset’s cerebral cortex. “The marmoset is a comparatively small primate, and it was the first time for us to perform head-fixed movement-task experiments using this animal. The marmoset is especially sensitive to stress, so there is always a fear that it will stop performing movement task. We repeatedly had to go through numerous technical trial-and-errors before we successfully performed an experiment.” What contributed greatly to this success was the training apparatus specifically designed and developed for marmoset use, and a training method which involved making the training more difficult precisely in tandem with the extent that the marmoset became used to the assigned task. Especially useful was the idea to have the marmoset wear a specially designed training “jacket,” which enabled smoother training performance. Dr. Masamizu discovered this while reading a scientific paper outside his field, related to medical-drug administration. The jacket was realized by Dr. Masamizu’s technical assistants on the basis of illustrations provided in the text. “Working with an animal that had no manual entailed step-by-step changes and improvements along the way. We are confident that our results are the first step in a new direction for elucidating the mechanisms of human cognition and movement, as well as the fundamentals of neuronal networks.”
Neuronal fibers, rich in potential for novel developments in a range of fields, from medical application to artificial intelligence
While Dr. Masamizu has continued with his basic research to pinpoint healthy brain activities, he is also challenging applied research. This includes the creation of neuronal fibers, plus the establishment of technologies for the implantation of these fibers into the brain for functional recovery. An ordinary neuron has one extension called the axon (as well as others called dendrites). The axon extends in a specific direction, and exchanges information with other neurons. When existing neurons are damaged in their original site, they lose their functions, as the axons do not extend in their specific directions. This has made the transplants of neuronal cell suspensions very difficult. Dr. Masamizu seeks to create neuronal fibers that maintain their orientation and can be transplanted within the brain. Currently, brain recovery is difficult when neurons are transplanted into areas in the brain damaged due to stroke, etc., especially depending on the width of the transplant area and how much time has elapsed since the damage occurred. In the future, if Dr. Masamizu can successfully develop neuronal fibers, it is hoped that a transplant that enables a bypass (detour) of the damaged area could lead to brain recovery (Fig. 1).

Fig. 1 Image diagram of bypassing damaged areas using the transplant of the neuronal fiber
If the implant of the neuronal fiber becomes possible, damaged brain areas beyond recovery can be bypassed, and new neurocircuits can be created. This could enable recovery of brain functions.
[Left image] In the case where information flows in the order A → B → C, when B is damaged, C cannot function.
[Right image] With the transplant of the neuronal fiber, B can be avoided, and a novel neuronal circuit is created
Dr. Masamizu also seeks ways to guide new discoveries with his research using in vivo calcium imaging. “We are working on a new approach, and that is to create new circuits in the brain with the transplantation of neuronal fibers, as well as to observe brain activity during that approach using the in vivo calcium imaging techniques that we have previously developed and refined. Recent developments in artificial technology research have also been performed using hints and suggestions from actual brain activity. With the construction of novel neuronal circuits, if we can see changes in brain functions as a result, it should be easy to incorporate these within mathematical models. We look forward to making contributions to this research area as well.”
Dr. Masamizu and his lab have thus continued to generate new and productive results in both basic and applied research. He says that his motivations for research have spurred his continued movement along paths that are not always the accepted and “correct” ways. “The brain is extremely complicated, and searching within its dark passageways can often be difficult and frustrating. But it is its unpredictability that makes experimentation and measurement, as well as making novel discoveries, so interesting and worthwhile.” Dr. Masamizu continues to perform his challenging work to unlock and solve puzzles of the brain. We can all eagerly look forward to more new and exciting research discoveries from him and his team into the future.
Using living cells to understand how neurons speak to one another
Our brains are complex organs with intricate networks of neurons. Day in and day out, neurons are consistently active within various neural circuits in our brains. Though research on the function of the brain and its neurons is rapidly advancing, many areas remain unexplored. For instance, the mechanisms underlying communication between neurons is yet to be elucidated.
Associate Professor Saitoh is engaged in research to “see” the live movements of molecules within neurons. “Neurons communicate with surrounding cells using a molecular ‘language,’ similar to how people use words to communicate with each other,” he says. “My research focuses on ‘how cells speak.’ I observe and analyze the molecular dynamics that take place within living cells (i.e., live cell imaging of neurons) to understand how cells use their words, speak to one another, and have their intentions communicated.”
In the life sciences, while molecular biology and biochemistry deal with molecules from inactive cells that have been purified or fixed to investigate the function and distribution of specific molecules, physiology deals only with living and functioning cells. Dr. Saitoh, who specializes in research on the functional analysis of the central nervous system, believes that understanding the movement of molecules within neurons will help elucidate the mechanisms that are involved in the development of diseases and disorders of the neural circuitry.
The successful development of a fluorescent sensor for the visualization of cAMP, an important molecule for the expression of cellular functions
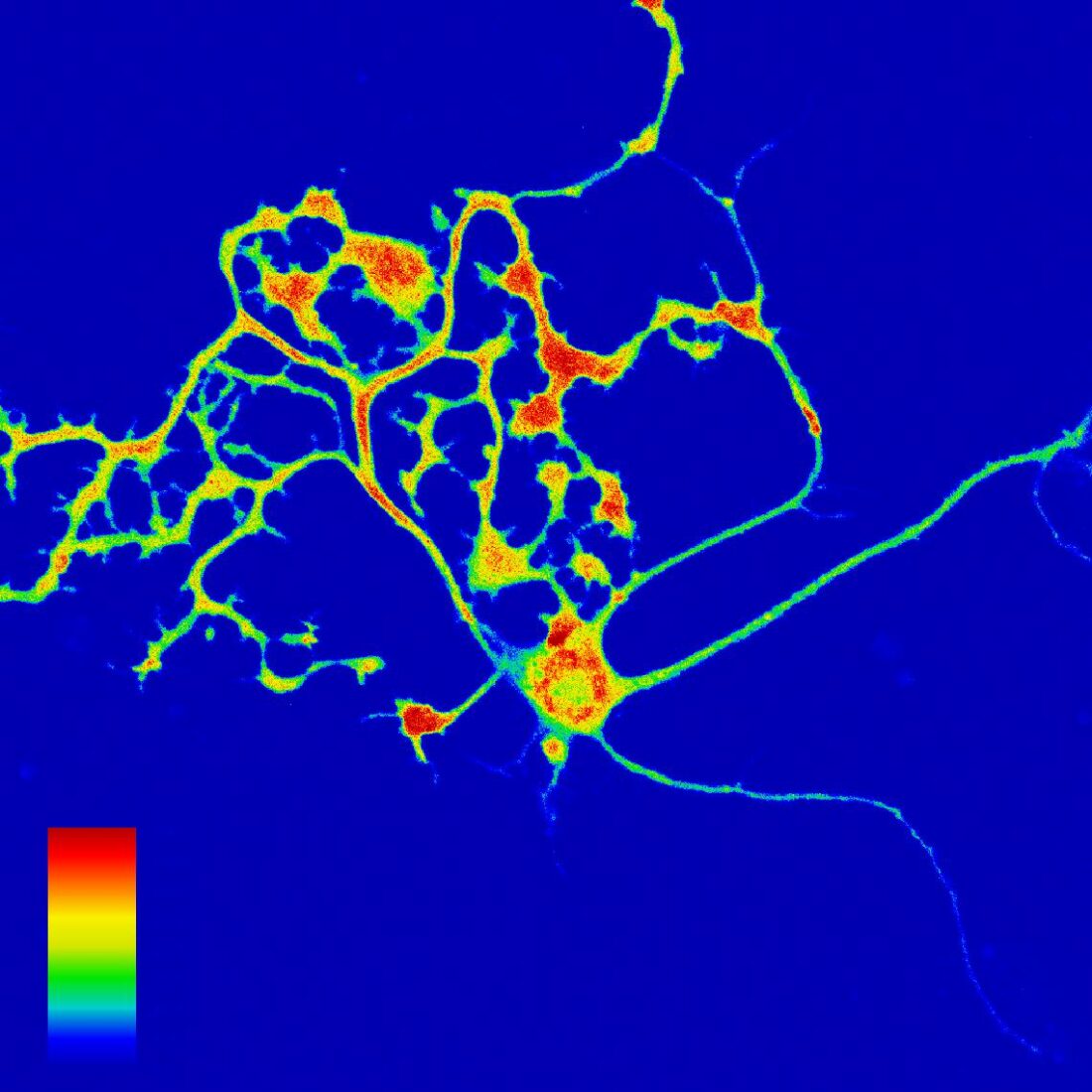
In particular, Dr. Saitoh is passionate about exploring the real-time imaging of cAMP (cyclic adenosine monophosphate)—a molecule involved in learning and memory formation. It is a second messenger that activates intracellular signal transduction systems. He observes, in real-time, the rise and fall of cAMP concentration levels in the neurons, and tracks the regions in which these fluctuations occur, the degree of fluctuations, and the timing at which they occur. “To observe the movement of colorless and transparent molecules in living cells through optical microscopes, it is necessary to apply fluorescence to specific molecules. Since cAMP itself does not fluoresce, we attached a fluorescent protein that can bind to cAMP( Figure 1 ).” Based on the intensity of the fluorescence of the fused protein, Dr. Saitoh succeeded in visualizing the concentration of cAMP in neurons ( Figure 2 ). This “development of a cAMP-specific fluorescent sensor” is expected to lead to new neurofunction-related discoveries.
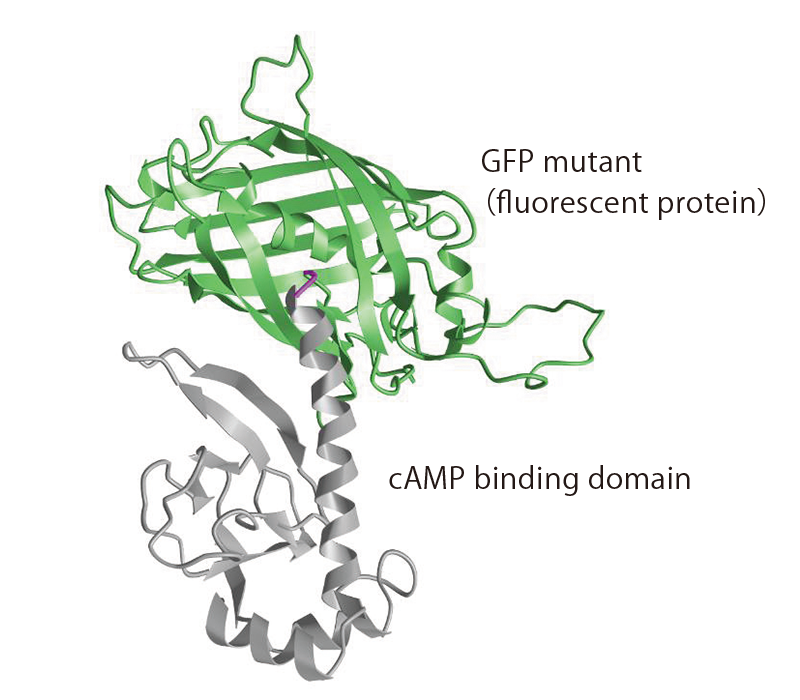
(Figure 1) Predicted steric structure of cAMP fluorescent indicator gCarvi
(Figure 2) Cultured Purkinje cells expressing gCarvi
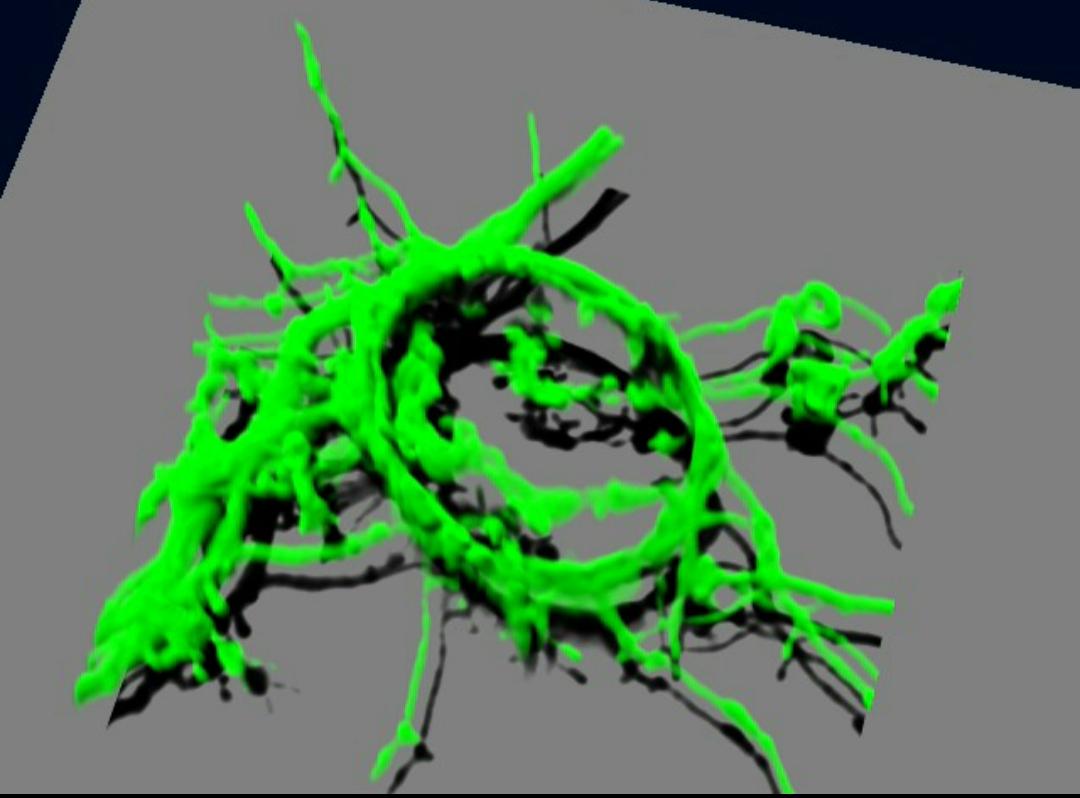
Furthermore, Dr. Saitoh has managed to culture the giant presynaptic terminals found in the brainstem: the calyx of Held synapse ( Figure 3 ), because almost all presynaptic terminals are structurally extremely small and difficult to observe under optical microscopes. Based on these successful experiments, he has unraveled new possibilities for the imaging of presynaptic terminals under optical microscopes.
According to Dr. Saitoh, these studies will contribute to our understanding of synaptic transmission regulatory systems, such as memory formation. “During the learning and memory formation process, long-term changes or long-term plasticity occurs as part of synaptic transmission. Among such long-term changes, those that last more than a day are grouped under late long-term plasticity—which is considered necessary for forming long-term memories. This late long-term plasticity will likely require an increase in cAMP concentration in the neurons’ nucleus. However, till date, no researcher has managed to observe this phenomenon. I believe my research will help uncover the reality of such a phenomenon,” muses Dr. Saitoh.
(Figure 3) Cultured Calyx expressing GFP
Dr. Saitoh also applied for a patent titled “cAMP Indicator and Its Manufacturing Method.” The findings of his research have reached a milestone, in that they are potentially applicable not only to neurons, but also to cells constituting other tissues. Furthermore, by analyzing the movement of intracellular cAMP via “GPCRs,” i.e., signal receptors on the cell surface, he hopes to contribute to research on drug discovery and target GPCRs related to various diseases.
Research on neural circuit homeostasis associated with Alzheimer’s disease: The way forward
Dr. Saitoh’s research is not only confined to cellular units, but also to neural circuits formed by neurons. He remarks, “While neural circuits are formed in cultures, the criterion to determine their formation is to maintain a stable spontaneous firing frequency. In cultured hippocampal neural circuits, the frequency is neither 10 Hz nor 0.1 Hz, but always at about 1 Hz. I believe that cAMP may be involved in the maintenance of homeostatic firing. However, the quantitative, temporal, and spatial cAMP dynamics are not yet known, and I would like to elucidate them using real-time cAMP imaging techniques.”
Dr. Saitoh adds that it is essential to understand the mechanism of neuronal circuit homeostasis to solve the mysteries of brain diseases. “Alzheimer’s disease, a brain disease, is caused due to the accumulation of amyloid-β, which leads to the death of neurons. Even if some neurons die or have defects in synaptic transmission, they should be able to readjust their circuits through the mechanism of homeostatic firing. By focusing on cAMP and elucidating the mechanism of enhancing neuronal homeostasis, I hope to advance my research to develop drugs that can prevent dementia,” he adds.
Dr. Saitoh has been steadily accumulating tangible results despite the many obstacles he has encountered along the way. He remarks that the joy of conducting research is closely linked to solving problems one by one and proving his hypotheses. “I am so fascinated by basic research that I cannot think of following any other path but that of a researcher,” he says, then adds with a smile, “I am making efforts to convey this fascination to my students. It would be a great honor for me if they contributed and enjoyed working in my field of research.” The enthusiasm and joy with which Dr. Saitoh hopes for his students’ success and the fruits of the development in his research field is truly inspiring.
