Observing brain activities with the aim of illuminating its functions
Neurophysiology is the scientific domain that investigates brain activities, including memory and learning. The aim is to determine brain-cell connection circuits (neurocircuits). Since “brain functions” is a broad term, this domain encompasses a wide range of fields, from basic psychology to cognitive neuroscience.
The development of observation methods is indispensable for the growth of this research domain. The key is to be able to capture the “real-time” state of the brain in more and more detail while it is actively working. Making those parts of the brain that are still largely unexplained visible is linked with the finding of novel facts. Dr. Masamizu, who specializes in neurophysiology, uses in vivo calcium imaging, a technique established around 2005, to explore and discover the true nature of the brain and its numerous mysteries. “We look at areas of the brain performing processing when an animal is performing a cognitive task, to determine how its brain cells (neurons) are acting. In in vivo calcium imaging, fluorescent calcium sensor proteins are used to monitor neural activity, which we then observe using a two-photon microscope and a macro zoom/multipoint laser scanning confocal microscope (Photo 1). This in vivo calcium imaging makes it easy to observe long-term activity changes in the same neurons. It is thus excellent for observing brain activity in animals used to perform experiments. Using this technique, we are clarifying neurocircuit networks involved in learning and memory, and more.”

Dr. Masamizu has been fascinated with the brain since his childhood. He selected a career as an academic researcher in order to learn and help unlock the principles of the brain. In his years as a graduate student, his research focused on the specialized field of neuroembryology, which studies the formation and development of the brain from the earliest stages. After he received his Ph.D., he moved to the field of neurophysiology. “I had a strong desire to know more and more about the brain, and thus studied brain development processes. However, as I pursued my research, I became aware of the need to know how the brain acts in order to acquire brain functions. This was the spark that led to my moving on to neurophysiology.”
Edging closer to new brain facts through step-by-step technological development
Dr. Masamizu uses in vivo calcium imaging to study rodents and primates. Their team was the first worldwide to determine that memory of learned movements is stored in a deep layer of the cerebral cortex. “The cortex has a six-layer structure. Prior research had observed layer 2/3 nearest the surface using in vivo calcium imaging. We made the special arrangement, which enabled the specific expression of fluorescent calcium sensors, used in in vivo calcium imaging, in deep layers. This enabled us to make observations of activity in deeper layers than before. As a movement task for a mouse, we devised an experiment where the mouse would receive water when pulling a lever for a fixed time. As result, we discovered that, in the late stage of learning, there was unique activity within layer 5 of the cerebral cortex motor area. We suggested that the neurons there might be responsible for motor-skills memory.”
Dr. Masamizu aims to reveal more about human brain functions. Using the marmoset—a primate that more closely resembles humans—to perform movement task experiments, he has also monitored neuronal activities in the marmoset’s cerebral cortex. “The marmoset is a comparatively small primate, and it was the first time for us to perform head-fixed movement-task experiments using this animal. The marmoset is especially sensitive to stress, so there is always a fear that it will stop performing movement task. We repeatedly had to go through numerous technical trial-and-errors before we successfully performed an experiment.” What contributed greatly to this success was the training apparatus specifically designed and developed for marmoset use, and a training method which involved making the training more difficult precisely in tandem with the extent that the marmoset became used to the assigned task. Especially useful was the idea to have the marmoset wear a specially designed training “jacket,” which enabled smoother training performance. Dr. Masamizu discovered this while reading a scientific paper outside his field, related to medical-drug administration. The jacket was realized by Dr. Masamizu’s technical assistants on the basis of illustrations provided in the text. “Working with an animal that had no manual entailed step-by-step changes and improvements along the way. We are confident that our results are the first step in a new direction for elucidating the mechanisms of human cognition and movement, as well as the fundamentals of neuronal networks.”
Neuronal fibers, rich in potential for novel developments in a range of fields, from medical application to artificial intelligence
While Dr. Masamizu has continued with his basic research to pinpoint healthy brain activities, he is also challenging applied research. This includes the creation of neuronal fibers, plus the establishment of technologies for the implantation of these fibers into the brain for functional recovery. An ordinary neuron has one extension called the axon (as well as others called dendrites). The axon extends in a specific direction, and exchanges information with other neurons. When existing neurons are damaged in their original site, they lose their functions, as the axons do not extend in their specific directions. This has made the transplants of neuronal cell suspensions very difficult. Dr. Masamizu seeks to create neuronal fibers that maintain their orientation and can be transplanted within the brain. Currently, brain recovery is difficult when neurons are transplanted into areas in the brain damaged due to stroke, etc., especially depending on the width of the transplant area and how much time has elapsed since the damage occurred. In the future, if Dr. Masamizu can successfully develop neuronal fibers, it is hoped that a transplant that enables a bypass (detour) of the damaged area could lead to brain recovery (Fig. 1).

Fig. 1 Image diagram of bypassing damaged areas using the transplant of the neuronal fiber
If the implant of the neuronal fiber becomes possible, damaged brain areas beyond recovery can be bypassed, and new neurocircuits can be created. This could enable recovery of brain functions.
[Left image] In the case where information flows in the order A → B → C, when B is damaged, C cannot function.
[Right image] With the transplant of the neuronal fiber, B can be avoided, and a novel neuronal circuit is created
Dr. Masamizu also seeks ways to guide new discoveries with his research using in vivo calcium imaging. “We are working on a new approach, and that is to create new circuits in the brain with the transplantation of neuronal fibers, as well as to observe brain activity during that approach using the in vivo calcium imaging techniques that we have previously developed and refined. Recent developments in artificial technology research have also been performed using hints and suggestions from actual brain activity. With the construction of novel neuronal circuits, if we can see changes in brain functions as a result, it should be easy to incorporate these within mathematical models. We look forward to making contributions to this research area as well.”
Dr. Masamizu and his lab have thus continued to generate new and productive results in both basic and applied research. He says that his motivations for research have spurred his continued movement along paths that are not always the accepted and “correct” ways. “The brain is extremely complicated, and searching within its dark passageways can often be difficult and frustrating. But it is its unpredictability that makes experimentation and measurement, as well as making novel discoveries, so interesting and worthwhile.” Dr. Masamizu continues to perform his challenging work to unlock and solve puzzles of the brain. We can all eagerly look forward to more new and exciting research discoveries from him and his team into the future.
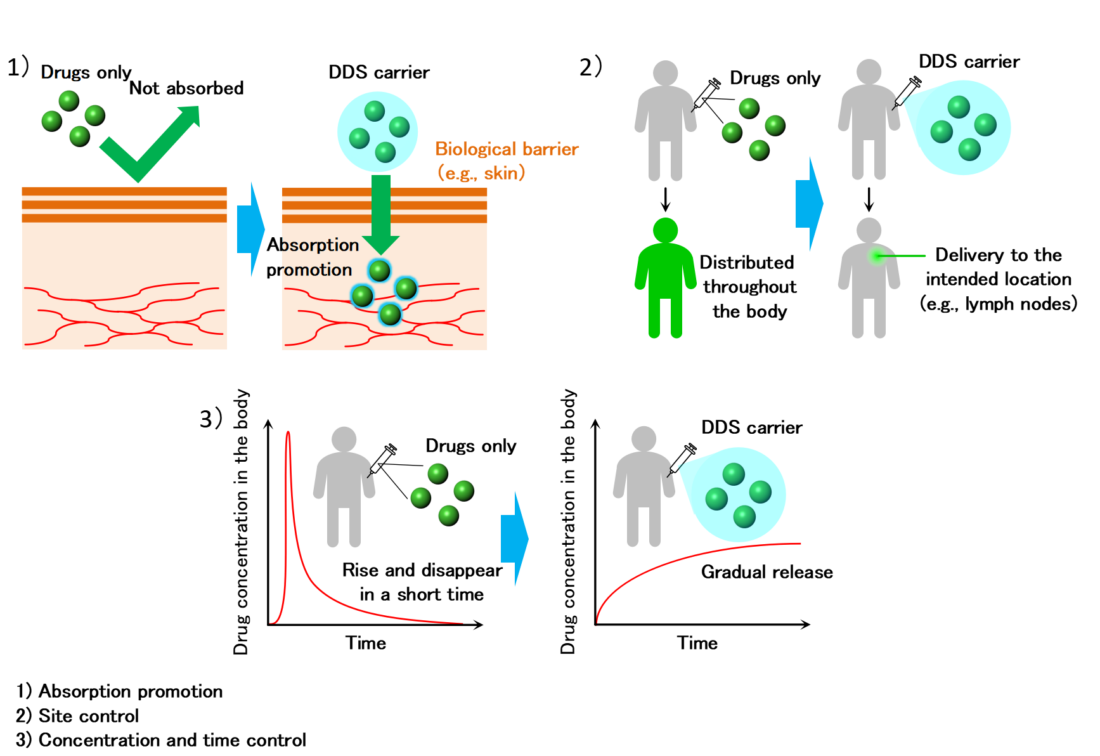
Controlling the site, amount, and time to maximize the efficacy of pharmaceuticals
The efficacy of medications depends on how and where they are administered. While there are many methods available for administering drugs, ranging from oral administration to injections, at times, drugs affect unrelated areas of the body, resulting in side effects. The drug delivery system (DDS) is a technology that addresses such drawbacks posed by medications.
DDS, a technology that delivers the appropriate amount of a pharmaceutical’s active ingredients to the appropriate site at the right time, not only increases its therapeutic effect but also decreases the stress on the patients by reducing side effects and required doses.
“I have been studying DDS with an engineering-based approach for over ten years,” says Associate Professor Dr. Tahara. “I have been working on developing and evaluating new materials (gels and emulsions) that can be applied in transdermal delivery, immunity DDS (vaccine, cancer immunity), and regenerative medicine.” He predicts that research on DDS based on biochemical engineering will undoubtedly expand. “In recent years, not only are researchers working on the transformation of the molecular structure of the drug substance itself, but also actively developing DDS carriers (e.g., fine particles) to encapsulate the drugs. Various approaches to DDS are being developed through the fusion of a wide range of fields, including science and engineering, medicine, dentistry, and pharmacy.”
Dr. Tahara stands at the forefront of a rapidly developing research field. His motivation to become a DDS researcher stems from his engagement in research during his student years concerning transdermal delivery methods, which allow drugs to penetrate the skin. Since transdermal delivery of drugs cause less stress on the patients, the pharmaceutical industry has been enthusiastically engaged in developing efficient transdermal delivery technologies. As Dr. Tahara explains, “People with diabetes administer insulin for treatment through injection, which works to lower the blood sugar levels. However, since the stress from the injections or needles for the patients is not insignificant, the development of transdermal delivery, such as an ointment, is desired. However, the corneum structure of the outermost layer of one’s skin has a highly hydrophobic structure. Thus, pharmaceuticals consisting of proteins such as insulin cannot be easily made into effective ointments as they are more easily soluble in water and have a high molecular weight. Back then, a new technology developed in our lab that produces nano-sized particles by coating the hydrophilic protein with a hydrophobic surfactant was found to be effective.” As a result, he succeeded in dispersing proteins, such as insulin, in an oil-based solution (a component that promotes percutaneous absorption).
The elucidation of the fundamental mechanisms is the basis of the widely applicable DDS research
DDS has the potential to play an active role in all aspects of drug administration. DDS carriers (fine particles) are also being developed for vaccine treatment, in which antigens derived from pathogens are administered to the body to enhance and memorize antigen-specific immunity (Figure 1). “We are currently working on the development of a DDS carrier for vaccine injections,” says Dr. Tahara. “We aim to elucidate efficient antigen delivery pathways and antigen information transmission mechanisms by observing differences in immune responses using DDS carriers that can efficiently deliver antigens to lymph nodes.”
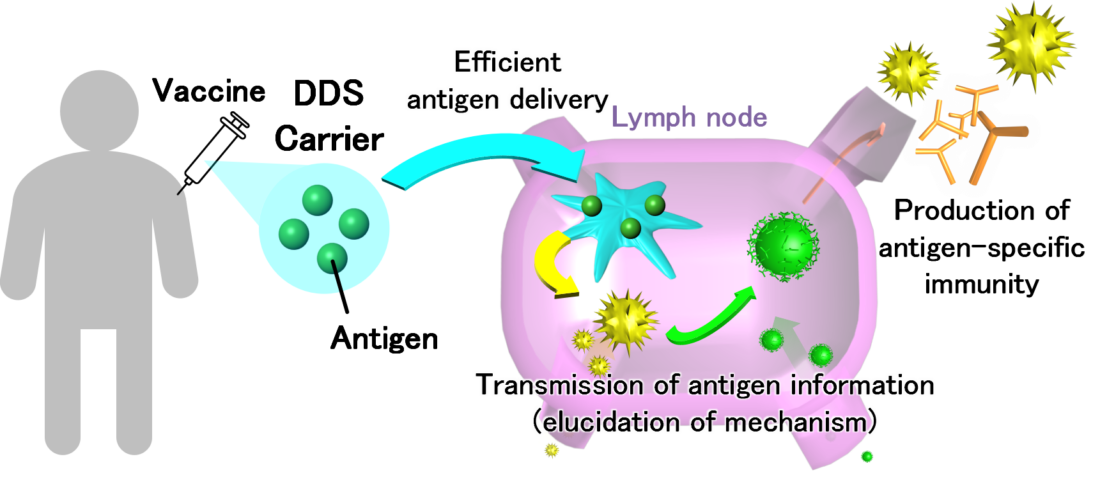
The basis of Dr. Tahara’s research, which is widely applicable across the medical field, is the elucidation of the basic mechanism concerning DDS. The aim of the Examination of Effective Administration Methods of Peptide Vaccines, as adopted by the COVID-19 Research Project, is to be prepared to present the optimal DDS when an important peptide sequence is discovered as an immunogen. “Each disease has a peptide sequence which is recognized as the key antigen,” says Dr. Tahara. “If we are able to determine the most appropriate administration method for the vaccine carrying that specific sequence, and if we can elucidate on the transmission mechanism of the antigen information after its administration, we could apply this not only to this novel Coronavirus but also to other new infectious diseases as well.”
Dr. Tahara has experience in researching regenerative medicine using biomaterials. In this field, which aims to regenerate lost organs and tissue, research is underway to transplant cells and scaffold materials based on glued cells cultured outside the body. Dr. Tahara explains, “I succeeded in creating a cell-adhesive gel of the same shape and size as a mouse femur during my post-doctoral research years. After that, via joint research, other doctors also succeeded in efficiently gluing fibroblasts and culturing it using this gel and inducing differentiation into osteoblasts.” Artificial 3D bone tissue transplantation plays an important role in treating patients with bone disease, such as those with skeletal deformities and joint disorders. “Understanding material adsorption in biological materials is an essential backdrop to this technology. Based on the experience I cultivated through this research, I wish to develop new gels with other functionalities and tackle areas in regenerative medicine,” he elucidates. Based on his experience, we can expect his findings to be applied in regenerative medicine through chemical engineering.
Advancing the way to practical application by utilizing pharmaceutical additives
What is particularly noteworthy about Dr. Tahara, a researcher in the field of basic research, is that his approach to research considers and involves industry-academia collaboration. “I am trying to develop a transdermal delivery system and DDS carriers by primarily combining pharmaceutical additives which their safety has been confirmed,” he states. “I use medical-grade ingredients with confirmed safety so that this can become a viable product as soon as possible.” While he pursues basic research, he is always consciously devising ways that will lead to practical use.
Dr. Tahara joins academic associations in fields other than his own to find new research themes. “As I participate in such groups, I get to know researchers in those fields personally. When we share information about our challenges and pursuits, sometimes we realize that research in our field could potentially resolve the challenges in others.” Medical researchers and researchers from the dental field have currently reached out for collaboration in joint research, each in their respective fields. We cannot keep our eyes off Dr. Tahara’s research activities, as he aims to contribute to society through DDS research.
Conceptual diagram of DDS
Reconstructing original function of tissues by applying physical stimuli.
Regenerative medicine refers to medical treatment that base on the self-healing ability of our body to regenerate tissue structures and their functions which are lost due to illness or injury. Although the research using iPS cells is well known, other advances in research in the field of regenerative medicine are also remarkable. In particular, the development of biomaterials having optimal biocompatibility has drawn worldwide attention.

“In the study of biomaterials, my target is regeneration of tissues related to movements of the human body such as bone, cartilage, muscles and nerves.” Prof. Morita has been approaching to create biomaterials with an altogether new approach combining medicine with engineering, by leveraging his research career in the fields of mechanical engineering and electrical engineering.
“The field of research regarding cell biology including genes and molecular structures, is of course important, but since I have an engineering background, I would like to contribute to regenerative medicine in my own way, making full use of my expertise in engineering,” said Prof. Morita. The foundation of his research is the tissue regeneration and cell activation by physical stimulation and the development of functional biomaterials.
“For example, during joint motions such as walking, cartilage is subject to weight bearing, so mechanical functions such as shock absorption and load support are required for the regenerated cartilage tissue. In this way, the musculoskeletal tissues function in response to physical stimuli. Therefore, unlike normal cell culture, cells are grown by applying electrical and mechanical stimuli that reproduce a pericellular environment in the objective living tissue. As a result, we expect that the regenerated tissue obtain the original functions of the living tissue.”
The purpose of medical care is not just to treat, but also to improve the quality of life of patients with reconstructing original tissue function. There have been great expectations of “biomaterials” created by Prof. Morita, which can derive optimal conditions for regeneration and enable to construct functional tissues more similar to the living tissues.
An innovative functional scaffold promotes the regeneration of cartilage and bone.
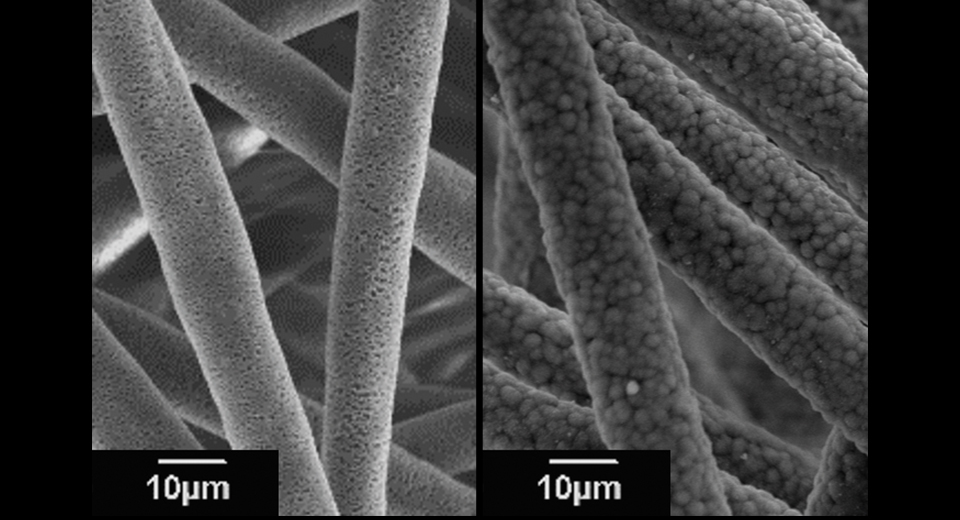
Once damaged, cartilage tissues do not heal itself well. This is because cartilage tissues do not have any blood vessels and thus, it is difficult to supply nutrients necessary for cure. Accordingly, there have been increased expectations for transplanting treatment using “cultured cartilage” prepared with isolated chondrocytes from natural cartilage or iPS cells. Prof. Morita engages in innovative scaffold development that can accelerate to regenerate cartilage tissues with superior mechanical properties.
“In cultured cartilage, the extracellular matrix produced by the cells limits the nutrient supply, reducing the activity of chondrocytes. Therefore, we have developed a PLLA (poly-L-lactic acid) microfiber scaffold with a porous hollow structure by electrospinning (see Photo 1). The culture medium penetrates through a large number of pores (small holes), maintaining cell activity.”
Electrospinning is a technology that is applied to fabricate nano/microfibers by applying a voltage to polymer solution using an electrospinning equipment (see Photo 2). By adjusting the spinning conditions, nano-scale microstructures can be controlled, and it is also used in the development of various materials for many tissues other than cartilage tissue.
In the field of bone regeneration, Prof. Morita is currently pursuing research on scaffolds to which dicalcium phosphate anhydrous (DCPA) are added (see Photo 3). “There is a research where the regeneration of autologous bone is encouraged by using bioabsorbable materials such as PLLA, but this poses some problems that it exhibits poor cell adhesion, and since mechanical properties are not equivalent to those of natural bone, it takes long period to regenerate. Thus, in concurrence with the addition of apatite precursors such as DCPA nanoparticles, which are the basis for bone formation, we made porous fibers. As a result, we succeeded in promoting prompt and uniform apatite formation on the fiber.”
Furthermore, in the process of bone regeneration, a cell environment equivalent to that of natural bone is quickly established in scaffolds coated with apatite, expecting the acceleration of the migration and adhesion of cells. Moreover, due to the specific surface area increased by porous structures, rapid bioabsorption of the fiber scaffold are also expected after the regeneration of autologous bone, enabling the repair of bone defects with promptly and newly formed autologous bone. As mentioned above, since the cells are activated by mimicking the bone environment, the promotion of bone regeneration is expected.
In addition, as the studies which further take an advantage of bone properties, Prof. Morita developed PLLA nanofiber scaffolds doped with barium titanate (BTO) nanoparticles, which are piezoelectric materials. “When forces are loaded on bone, electric potentials are generated, and osteoblasts are stimulated, resulting in the bone formation. Therefore, we thought that if we apply piezoelectric materials that generate potential and surface charge when forces are applied to scaffolds, due to the impacts during walking, bone-like environment within scaffolds will be reproduced. We have been making continuous improvements by searching for piezoelectric materials that are harmless to the human body and by controlling the amounts of piezoelectric particles distributed in the nanofibers,” stated Prof. Morita who has been proactively working toward the practical use of these research achievements. The prompt repair of bone defects can bring to our lives significant benefits such as early discharge of patients from hospitals and reduction of healthcare costs.
The key to bone regeneration is to reproduce an in vivo environment.
Diversified fields of expertise are the source of research.
As a result of the research conducted by Prof. Morita, such as reproduction of bone properties through the addition of effective materials and reproduction of bone environment by using materials with apatite precipitation ability, it has become clear that regeneration can be promoted by designing and imitating the in vivo environment. A broad perspective of Prof. Morita who has experiences in electricity in the engineering field is indispensable for creating an in vivo environment.
“Because I have undertaken research in a variety of fields such as electricity and machinery, I have been able to think flexibly using my engineering expertise without being bound by stereotypes. Moreover, since various engineering technology and developed materials are used for tissue engineering and medical treatment, there is an inseparable link between medicine and engineering. Because I specialize in both fields, I believe I can contribute to research of tissue regeneration with multi-disciplinary approach. In the situation of declining birthrate and aging, the medical target is mainly the elderly, but I would also like to promote research focused on younger generations.”
Research achievements with an approach combining medicine with engineering could hold immense potential in regenerative medicine. Amid the ongoing technological development including artificial intelligence (AI) which aims for labor savings in all areas, the research conducted by Prof. Morita to challenge regenerative medicine that promotes “self-healing activity” will remind us the potential of human power.