Interview with researchers 3 Creation of biomaterials and their optimal application by fusion of medicine with engineering

- Interview Yusuke Morita
- Professor, Faculty of Life and Medical Sciences, Department of Biomedical Engineering
Reconstructing original function of tissues by applying physical stimuli.
Regenerative medicine refers to medical treatment that base on the self-healing ability of our body to regenerate tissue structures and their functions which are lost due to illness or injury. Although the research using iPS cells is well known, other advances in research in the field of regenerative medicine are also remarkable. In particular, the development of biomaterials having optimal biocompatibility has drawn worldwide attention.

“In the study of biomaterials, my target is regeneration of tissues related to movements of the human body such as bone, cartilage, muscles and nerves.” Prof. Morita has been approaching to create biomaterials with an altogether new approach combining medicine with engineering, by leveraging his research career in the fields of mechanical engineering and electrical engineering.
“The field of research regarding cell biology including genes and molecular structures, is of course important, but since I have an engineering background, I would like to contribute to regenerative medicine in my own way, making full use of my expertise in engineering,” said Prof. Morita. The foundation of his research is the tissue regeneration and cell activation by physical stimulation and the development of functional biomaterials.
“For example, during joint motions such as walking, cartilage is subject to weight bearing, so mechanical functions such as shock absorption and load support are required for the regenerated cartilage tissue. In this way, the musculoskeletal tissues function in response to physical stimuli. Therefore, unlike normal cell culture, cells are grown by applying electrical and mechanical stimuli that reproduce a pericellular environment in the objective living tissue. As a result, we expect that the regenerated tissue obtain the original functions of the living tissue.”
The purpose of medical care is not just to treat, but also to improve the quality of life of patients with reconstructing original tissue function. There have been great expectations of “biomaterials” created by Prof. Morita, which can derive optimal conditions for regeneration and enable to construct functional tissues more similar to the living tissues.
An innovative functional scaffold promotes the regeneration of cartilage and bone.
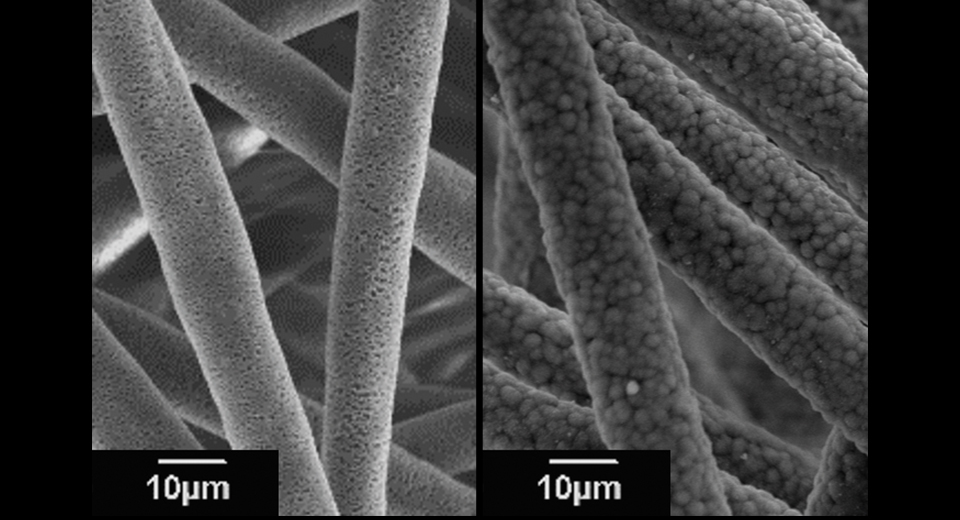
Once damaged, cartilage tissues do not heal itself well. This is because cartilage tissues do not have any blood vessels and thus, it is difficult to supply nutrients necessary for cure. Accordingly, there have been increased expectations for transplanting treatment using “cultured cartilage” prepared with isolated chondrocytes from natural cartilage or iPS cells. Prof. Morita engages in innovative scaffold development that can accelerate to regenerate cartilage tissues with superior mechanical properties.
“In cultured cartilage, the extracellular matrix produced by the cells limits the nutrient supply, reducing the activity of chondrocytes. Therefore, we have developed a PLLA (poly-L-lactic acid) microfiber scaffold with a porous hollow structure by electrospinning (see Photo 1). The culture medium penetrates through a large number of pores (small holes), maintaining cell activity.”
Electrospinning is a technology that is applied to fabricate nano/microfibers by applying a voltage to polymer solution using an electrospinning equipment (see Photo 2). By adjusting the spinning conditions, nano-scale microstructures can be controlled, and it is also used in the development of various materials for many tissues other than cartilage tissue.
In the field of bone regeneration, Prof. Morita is currently pursuing research on scaffolds to which dicalcium phosphate anhydrous (DCPA) are added (see Photo 3). “There is a research where the regeneration of autologous bone is encouraged by using bioabsorbable materials such as PLLA, but this poses some problems that it exhibits poor cell adhesion, and since mechanical properties are not equivalent to those of natural bone, it takes long period to regenerate. Thus, in concurrence with the addition of apatite precursors such as DCPA nanoparticles, which are the basis for bone formation, we made porous fibers. As a result, we succeeded in promoting prompt and uniform apatite formation on the fiber.”
Furthermore, in the process of bone regeneration, a cell environment equivalent to that of natural bone is quickly established in scaffolds coated with apatite, expecting the acceleration of the migration and adhesion of cells. Moreover, due to the specific surface area increased by porous structures, rapid bioabsorption of the fiber scaffold are also expected after the regeneration of autologous bone, enabling the repair of bone defects with promptly and newly formed autologous bone. As mentioned above, since the cells are activated by mimicking the bone environment, the promotion of bone regeneration is expected.
In addition, as the studies which further take an advantage of bone properties, Prof. Morita developed PLLA nanofiber scaffolds doped with barium titanate (BTO) nanoparticles, which are piezoelectric materials. “When forces are loaded on bone, electric potentials are generated, and osteoblasts are stimulated, resulting in the bone formation. Therefore, we thought that if we apply piezoelectric materials that generate potential and surface charge when forces are applied to scaffolds, due to the impacts during walking, bone-like environment within scaffolds will be reproduced. We have been making continuous improvements by searching for piezoelectric materials that are harmless to the human body and by controlling the amounts of piezoelectric particles distributed in the nanofibers,” stated Prof. Morita who has been proactively working toward the practical use of these research achievements. The prompt repair of bone defects can bring to our lives significant benefits such as early discharge of patients from hospitals and reduction of healthcare costs.
The key to bone regeneration is to reproduce an in vivo environment.
Diversified fields of expertise are the source of research.
As a result of the research conducted by Prof. Morita, such as reproduction of bone properties through the addition of effective materials and reproduction of bone environment by using materials with apatite precipitation ability, it has become clear that regeneration can be promoted by designing and imitating the in vivo environment. A broad perspective of Prof. Morita who has experiences in electricity in the engineering field is indispensable for creating an in vivo environment.
“Because I have undertaken research in a variety of fields such as electricity and machinery, I have been able to think flexibly using my engineering expertise without being bound by stereotypes. Moreover, since various engineering technology and developed materials are used for tissue engineering and medical treatment, there is an inseparable link between medicine and engineering. Because I specialize in both fields, I believe I can contribute to research of tissue regeneration with multi-disciplinary approach. In the situation of declining birthrate and aging, the medical target is mainly the elderly, but I would also like to promote research focused on younger generations.”
Research achievements with an approach combining medicine with engineering could hold immense potential in regenerative medicine. Amid the ongoing technological development including artificial intelligence (AI) which aims for labor savings in all areas, the research conducted by Prof. Morita to challenge regenerative medicine that promotes “self-healing activity” will remind us the potential of human power.

