Research News: Novel Sustainable Electrochemical Method Converts Carbon Dioxide into Carbonaceous Materials
May 15, 2023
Researchers from Japan have developed a new method for the electrochemical reduction of carbon dioxide using high-temperature molten salts
Nations across the globe are striving relentlessly to reduce their net carbon footprint. Efforts are currently being undertaken worldwide for the depletion of greenhouse gases including carbon dioxide (CO2). To this end, a research team from Doshisha University, Japan has recently developed a novel method for converting CO2 into useful and commercially viable carbonaceous materials such as multi-walled carbon nanotubes with the help of sustainable electrochemistry.
Carbon dioxide (CO2) is a major greenhouse gas emitted through various types of human activities. In an effort to decrease humanity’s carbon footprint, scientists and policymakers across the globe are continuously trying to explore new methods for reducing atmospheric CO2 emissions and converting them into useful forms. In this regard, the electrochemical method of reducing CO2 to other carbonaceous forms like carbon monoxide, alcohols and hydrocarbon has gained considerable attention.
Against this backdrop, environmental researchers from Doshisha University, Japan led by Prof. Takuya Goto recently published a study in Electrochimica Acta on 10 July 2023 that demonstrated one such method for converting CO2 into multi-walled carbon nanotubes (MWCNT) using molten salts through sustainable electrochemistry. Their study was made available online on 22 April 2023, and included contributions from Dr. Yuta Suzuki from the Harris Science Research Institute and Mr. Tsubasa Takeda from the Department of Science of Environment and Mathematical Modeling.
Using a sustainable electrochemical technique, the research team facilitated the conversion of CO2 into MWCNT using LiCl-KCl melt. The molten salts were saturated with CO2 gas and semi-immersed nickel (Ni) substrate was used as electrode. The supplied CO2 was electrochemically converted to solid carbon at the end of the procedure. This green conversion occurred via a reduction reaction at the Ni electrode/LiCl-KCl melt/CO2 interface.
“The electrochemical reduction of CO2 on a Ni electrode in LiCl-KCl melt at 723 K was studied. Under high polarization, a super meniscus was formed at the three-phase interface of the Ni electrode/LiCl-KCl melt/CO2 gas, where the direct electrochemical reduction of CO2 to solid carbon progressed. Solid carbon was obtained in the wetted area of the Ni electrode as well as in the bulk molten salt via the electrochemical technique,” remarks Prof. Goto.
Subsequent characterization of the electrode-deposited carbon using electron microscopy techniques and elemental analysis revealed that the obtained carbonaceous material consisted of MWCNTs, commercially viable nanostructures, that were 30 – 50 nm in diameter. The team then varied the applied voltage and extended the reaction time, recording noticeable changes in the MWCNTs. The height of the generated MWCNTs increased after the electrolysis time was increased from 10 min to 180 min. “We studied the dependence of applied potential and electrolytic time on the morphology and crystallinity of the electrodeposited carbon. Based on our experimental results, we proposed a model for the formation of the MWCNTs on the Ni electrode,” highlights Prof. Goto.
The proposed model for the generation of MWCNTs from CO2 is described in three stages. The first stage involves the reduction of CO2 to carbon atoms at the Ni/LiCl-KCl melt/CO2 interface. During the second stage, the electrodeposited carbon atoms form Ni-C compounds (like NiC) on the surface of Ni electrode. Finally, when solubility limit of carbon in Ni-C compounds is reached, the cylindrical-shaped MWCNTs grow from the edge of the Ni-C compounds generated during the second stage.
In summary, the study identifies a novel process for sustainably converting CO2 into commercially useful carbonaceous materials. Moreover, the employed electrochemical process is environment friendly owing to the non-usage of fossil fuel. In addition, the use of high-temperature molten salts is unique because it enables the direct conversion of CO2 gas into MWCNTs.
“Our results indicate that CO2 can be converted into carbonaceous functional materials. By combining non-consumable oxygen-evolving anodes, this technique can contribute to the development of a carbon recycling technology that will not only solve global environmental problems but also play an important in carbon pricing economies. The material production process, which does not use fossil fuels, will help realize a sustainable society in the near future,” concludes an optimistic Prof. Goto.
We certainly hope his visions will be realized soon!
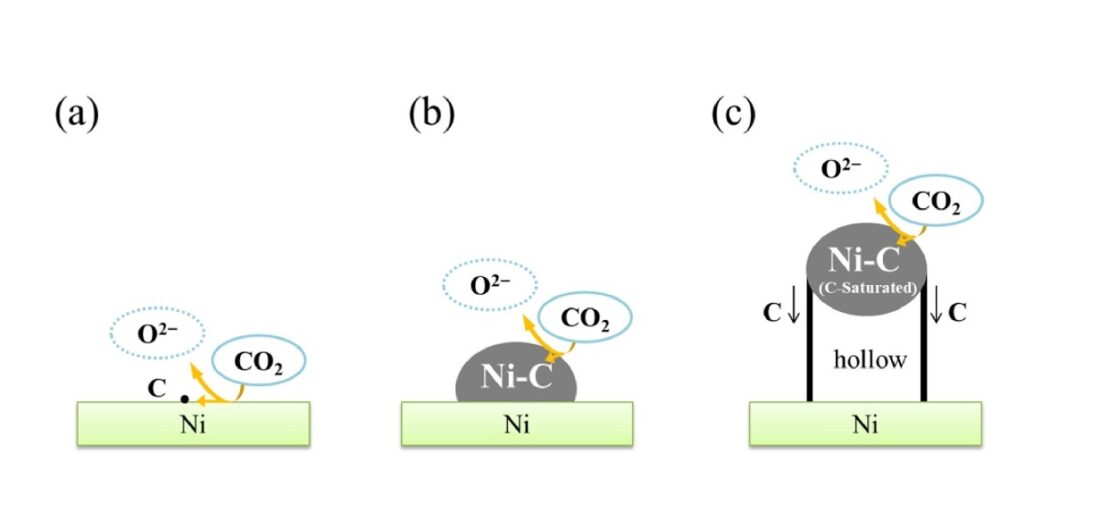
Schematic model depicting the stepwise formation of multi-walled carbon nanotube on a nickel substrate.
In light of the growing concerns surrounding global warming and carbon footprint, researchers from Japan came up with a technique that could contribute to the development of eco-friendly and sustainable carbon recycling.
Image courtesy: Takuya Goto from Doshisha University, Japan (https://doi.org/10.1016/j.electacta.2023.142464)
Image license: CC BY 4.0
Reference
| Title of original paper | Direct Electrochemical Formation of Carbonaceous Material from CO2 in LiCl-KCl Melt |
| Journal | Electrochimica Acta |
| DOI | 10.1016/j.electacta.2023.142464 |
Profiles

Takuya Goto
Professor, Faculty of Science and Engineering Department of Environmental Systems Science
Prof. Takuya Goto serves as a Professor at the Department of Science of Environment and Mathematical Modeling, Graduate School of Science and Engineering at Doshisha University, Japan. He obtained his Ph.D. in energy science from Kyoto University, Japan. Prof. Goto has over 80 publications to his credit. His laboratory primarily conducts research in the area of energy science, nuclear engineering, inorganic chemistry, and electrochemistry.

Yuta Suzuki
Assistant Professor, Harris Science Research Institute
Dr. Yuta Suzuki is the first author of this exciting paper on reducing CO2 emissions. He received his doctorate under the supervision of Prof. Goto.
Media contact
Organization for Research Initiatives & Development
Doshisha University
Kyotanabe, Kyoto 610-0394, JAPAN
CONTACT US
