Research News: Unveiling the Intricate Mechanisms Behind Oxysterol-Induced Cell Death
February 21, 2025
Researchers reveal that 25-hydroxycholesterol induces a particular type of cell death called ferroptosis, which could be implicated in several degenerative diseases
Oxysterols, molecules derived from cholesterol, play crucial roles in our bodies but can also contribute to various diseases.Now, researchers from Japan have discovered that 25-hydroxycholesterol (25-OHC) triggers a specific type of cell death called ferroptosis in nerve-supporting cells. The study reveals that 25-OHC disrupts cellular protective mechanisms and antioxidant systems. These findings could lead to new diagnostics and treatments for conditions, like amyotrophic lateral sclerosis, where elevated 25-OHC levels have been observed.
Oxysterols are a class of molecules derived from cholesterol via oxidation or as byproducts of cholesterol synthesis. Despite their relatively low concentration within our bodies, oxysterols are known to play many important biological roles, acting as transcriptional regulators, precursors for bile acid, and key players in brain development.
On the flip side, some pathologies are associated with imbalances in oxysterols. In particular, 25-hydroxycholesterol (25-OHC) has been shown to contribute to arteriosclerosis, cancer development, central nervous system disorders, dysregulated immune responses, and macular degeneration. Despite substantial efforts, the molecular mechanisms by which 25-OHC affects glial cells in diseases like amyotrophic lateral sclerosis (ALS) remain unclear.
Against this backdrop, a research team led by Professor Yasuomi Urano and Professor Noriko Noguchi from the Graduate School of Life and Medical Sciences, Doshisha University, Japan, explored the intricate roles of 25-OHC in inducing cell death, particularly in glial cells. Their paper was made available online on January 6, 2025, and was published in Volume 228 of Free Radical Biology and Medicine on February 16, 2025.
The researchers sought to clarify how 25-OHC contributes to cell death in Schwann cell cultures, which are essential supporting cells in the nervous system. Through extensive experimentation, they revealed that 25-OHC induces a specific type of cell death called ferroptosis—an iron-dependent process characterized by the accumulation of toxic fatty molecules within cells. “While 25-OHC is thought to be mainly associated with inducing apoptosis in various cell types, our findings highlight the importance of considering ferroptosis as a potential cell death mechanism triggered by a similar concentration of 25-OHC,” explains Urano.
More specifically, the team identified two key mechanisms for 25-OHC-induced ferroptosis. First, 25-OHC inhibits important cellular pathways involving the processing of SREBPs (or Sterol Regulatory Element-Binding Proteins), which normally help maintain lipid homeostasis and protect cells from metabolic imbalance. Second, it disrupts the cell’s natural antioxidant systems by reducing levels of a crucial protective enzyme called glutathione peroxidase 4(GPX4), leading to redox imbalances.
Interestingly, the researchers revealed that even at concentrations too low to directly cause cell death, exposure to 25-OHC makes cells more susceptible to ferroptosis. This result has significant implications for understanding how diseases like ALS might progress, as even modest elevations in 25-OHC levels could contribute to long-term cellular damage.
Together, the findings suggest several promising directions for future investigation, including the development of drugs that could block ferroptosis or prevent 25-OHC accumulation. Alternatively, one could also leverage oxysterols to make tumor cells more vulnerable. “Considering the promising potential of cancer therapy with ferroptosis inducers, 25-OHC is expected to enhance the efficacy of ferroptosis inducers as anticancer agents,” highlights Urano. Additionally, measuring 25-OHC levels could potentially serve as a biomarker for the early detection of various diseases, though this would require further validation.
Overall, this study represents a significant step forward in understanding how oxysterols contribute to both regular and pathological processes. With any luck, these newfound insights will let us develop innovative therapeutic and diagnostic strategies for challenging conditions.
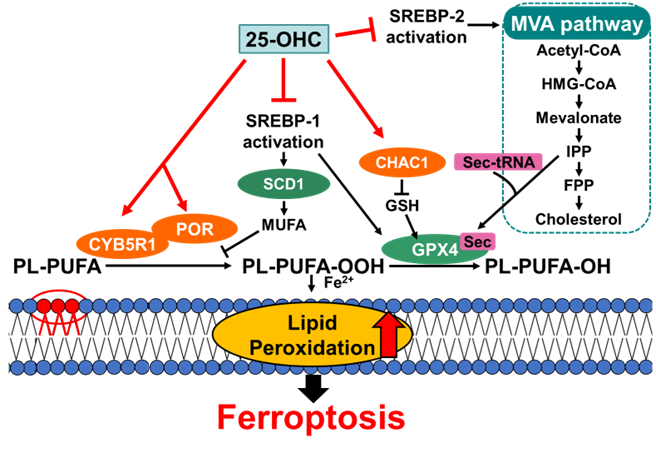
Overview of the proposed mechanism of 25-OHC-induced ferroptosis
This image summarizes the main findings of the study, showing the multifaceted effects that exposure to 25-hydroxycholesterol (25-OHC) has on Schwann cells.
Image courtesy: Professor Yasuomi Urano from Doshisha University, Japan
Image license: CC BY 4.0
Image link: https://www.sciencedirect.com/science/article/pii/S0891584925000103?via%3Dihub
Usage restrictions: You are free to share and adapt but credit must be given to the creator.
Reference
| Title of original paper | Downregulation of the SREBP pathways and disruption of redox status by 25-hydroxycholesterol predispose cells to ferroptosis |
| Journal | Free Radical Biology and Medicine |
| DOI | 10.1016/j.freeradbiomed.2025.01.010 |
Funding information
This work was supported in part by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) 19K07093 and 23K10902.
EurekAlert!
https://www.eurekalert.org/news-releases/1074198
Profile

Yasuomi Urano
Professor, Faculty of Life and Medical Sciences Department of Medical Life Systems
Professor Yasuomi Urano is a distinguished researcher at Doshisha University’s Graduate School of Life and Medical Sciences. He joined Doshisha University in 2009 as an Assistant Professor and was promoted to Associate Professor in 2018 and to Full Professor in 2024. His research focuses on cell death mechanisms, oxysterols, neuroscience, and lipid metabolism, with particular emphasis on cholesterols and their role in neuropathologies. He has published over peer-reviewed 40 research papers on these topics.

Noriko Noguchi
Professor, Faculty of Life and Medical Sciences Department of Medical Life Systems
Dr. Noriko Noguchi is a Professor at the Graduate School of Life and Medical Sciences at Doshisha University. She received her Ph.D. from the University of Tsukuba, Japan, in 1987. Her research focuses on elucidating the molecular mechanisms of lifestyle-related diseases such as Alzheimer’s disease, diabetes, and cancer, with a particular emphasis on oxidative stress. She has more than 38 years of experience as a researcher and has about 200 publications in peer-reviewed international journals.
Media contact
Organization for Research Initiatives & Development
Doshisha University
Kyotanabe, Kyoto 610-0394, JAPAN
CONTACT US
