Research News: “Plaque Preference: Why Are Alzheimer’s-Associated Amyloid- β Levels Low in the Cerebellum?”
February 26, 2021
Amyloid deposits usually occur in the cerebral cortex but not the cerebellum, and Doshisha University scientists have found out why
Amyloid-β deposits are an important aspect of Alzheimer’s disease pathology, and plaques formed of amyloid-β are usually observed in the cerebral cortex, but not the cerebellum. In a recent study, a team of scientists from Japan has provided evidence that the lower levels of amyloid-β deposition in the cerebellum arise from enhanced clearance of amyloid-β. This finding may help guide new efforts to prevent and treat Alzheimer’s disease.
Alzheimer’s disease is the most common cause of dementia in elderly people, and a hallmark feature of Alzheimer’s disease pathology is the formation of senile plaques composed of amyloid-β in brain areas that include the cerebral cortex, the hippocampus, and the amygdala. In contrast, the presence of senile plaques in the cerebellum is observed only in the most severe cases.
The reasons for the near-absence of amyloid-β plaques in the cerebellum are poorly understood, which motivated a team of scientists from Japan, led by Associate Professor Satoru Funamoto of Doshisha University, to find out why. As Dr. Funamoto explains, “it is very important to elucidate the mechanisms that suppress amyloid-β deposition in the cerebellum because knowledge of such mechanisms may contribute not just to our understanding of the pathogenesis of Alzheimer’s disease but also our understanding of how Alzheimer’s disease could be prevented or cured.” The results of this research appear in a paper published in a recent issue of Biochemical and Biophysical Research Communications.
To explore the mechanisms underlying low cerebellar amyloid-β deposition, the scientists worked with mice that were genetically modified so that they expressed the human form of the amyloid precursor protein, which plays an important role in the production of amyloid-β. As expected, these genetically modified mice developed more amyloid-β plaques in the cerebral cortex than in the cerebellum as they aged.
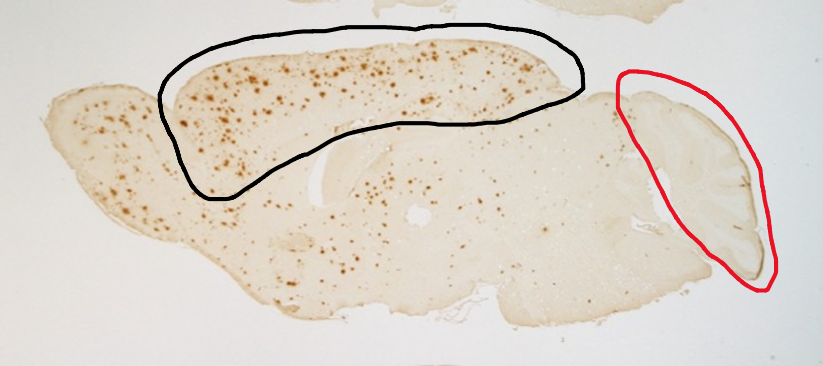
Immunostaining of the brain cross-section of genetically modified mice reveals that the amount of amyloid-β is higher in the cerebral cortex (shown in a black outline, indicated by the high number of red dots in the center to left regions) as compared to the cerebellum (indicated with the red outline)
However, when the scientists measured amyloid-β levels in the liquid called interstitial fluid that surrounds the cells, they found no difference between the amyloid-β levels of interstitial fluid from the cerebral cortex and the amyloid-β levels of interstitial fluid from the cerebellum. This means that there was likely no difference in the amount of amyloid-β being produced in the first place in both regions.
The scientists had to reconsider their hypothesis: what if similar amounts of amyloid-β were produced in both regions, but the clearance of amyloid-β was more efficient in the cerebellum than in the cerebral cortex? To test this possibility, the scientists conducted experiments that involved injecting the mice with amyloid-β proteins that carried fluorescent tags, which allowed the scientists to use microscopes to determine where the proteins went after injections. The scientists injected the fluorescently labelled amyloid-β directly into the cerebral cortex in some mice, and they injected it directly into the cerebellum in others. Interestingly, the area over which the fluorescently labelled amyloid-β spread within 2 hours of an injection was six times greater if it was injected into the cerebellum than if it was injected into the cerebral cortex. This finding is consistent with the hypothesis of enhanced clearance of amyloid-β from the cerebellum.
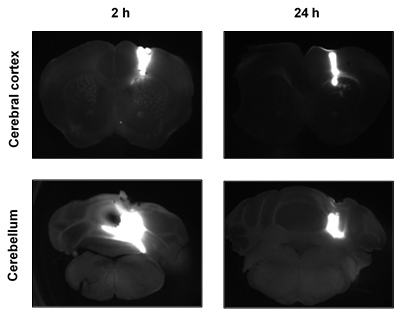
The spread of fluorescently labelled amyloid-β following injection into either the cerebral cortex (top) or the cerebellum (bottom). The protein diffused over a much larger area following injection into the cerebellum, suggesting more efficient clearance
Furthermore, over the 24 hours after an injection of amyloid-β into the cerebellum, the amount of amyloid-β remaining in the cerebellum decreased sharply, and much of the amyloid-β found its way to the deep cervical lymph nodes, which is where filtered matter in the body often ends up. In contrast, fluorescently labelled amyloid-β injected into the cerebral cortex maintained a presence in the cerebral cortex for up to 72 hours after the injection, and relatively little of it found its way to the deep cervical lymph nodes. These findings provide further evidence for enhanced clearance of amyloid-β from the cerebellum.
In summary, these findings indicate that differences in amyloid-β deposition levels between the cerebral cortex and cerebellum result from enhanced clearance of amyloid-β from the cerebellum. Dr. Funamoto notes that this finding is evidence that “the clearance or degradation of amyloid-β is more important than its synthesis in explaining how it accumulates,” and this suggests that amyloid-β clearance pathways may be “a new therapeutic target for treating Alzheimer’s disease.” These findings could therefore ultimately lead to new ways to prevent or treat Alzheimer’s disease.
Reference
| Title of original paper | A potential defense mechanism against amyloid deposition in cerebellum |
| Journal | Biochemical and Biophysical Research Communications |
| DOI | 10.1016/j.bbrc.2020.12.036 |
Funding information
This study was supported by Doshisha University and the Japan Agency for Medical Research and Development.
Profile

Associate Professor Satoru Funamoto works within the Faculty of Life and Medical Sciences of the Department of Medical Life Systems at Doshisha University.
His research interests include Alzheimer’s disease and proteins that play key roles in the disease, including amyloid-β.
He earned his Ph.D. at Hokkaido University, and he conducted research at the University of Tokyo between 2002 and 2007. His work has led to numerous papers, book chapters, and conference presentations and a patent.
Satoru Funamoto
Associate Professor, Faculty of Life and Medical Sciences, Department of Medical Life Systems
Contact
Doshisha University Industry Liaison Office
Kyotanabe, Kyoto 610-0394, JAPAN
CONTACT US
