Elucidating Liquid-Liquid Phase Separation Under Non-Equilibrium Conditions
January 26, 2026
Researchers examine the water/ethanol/butylparaben system to improve their understanding of localized liquid-liquid phase separation
Liquid-liquid phase separation (LLPS) is a peculiar phenomenon that occurs during crystallization. In a new study, a group of researchers from Doshisha University, Japan, have investigated the non-equilibrium phase behavior of localized LLPS driven by initial solute concentration and antisolvent addition rate in a ternary water/ethanol/butylparaben system. The present findings provide insights that may help regulate LLPS in turn potentially paving the way for improved product quality in the pharmaceutical, agrochemical, and food industries.
Crystallization is a well-explored natural phenomenon where atoms or molecules arrange themselves into highly organized solid forms called as crystals. This phenomenon has been widely utilized across pharmaceutical and agrochemicals industries, as well as in food industries, to form, separate, and purify pure crystalline materials. The process is usually considered a solid-liquid phase transition, but it can also involve liquid-liquid phase separation (LLPS). In LLPS, the solution separates out into solute-rich and solute-poor liquid phases. Crucially, this secondary phenomenon can significantly degrade product quality in terms of agglomeration, purity, and particle size. On the other hand, it can also help produce useful crystal morphologies and enhance impurity separation.
Scientists conventionally use phase diagrams to carry out crystallization processes by determining LLPS regions under equilibrium. However, crystallization usually also features non-equilibrium dynamics, especially during the addition of antisolvent—the liquid added to a solution to lower the solubility of a solute. In such scenarios, instabilities such as localized supersaturation and transient spinodal regions (thermodynamically unstable area on a phase diagram) can unexpectedly induce LLPS in one-phase regions of phase diagrams. Therefore, it is necessary to examine LLPS from a non-equilibrium viewpoint, in terms of kinetic factors like initial solute concentration and antisolvent addition rate.
To bridge this knowledge gap, a team of researchers from Japan, led by Professor Yoshiyuki Shirakawa from the Department of Chemical Engineering and Materials Science at Doshisha University and including Naoya Matsushima and Dr. Yuhei Tsugawa from the Graduate School of Science and Engineering at Doshisha University, Professor Mikio Yoshida from the Department of Chemical Engineering and Materials Science at Doshisha University, and Professor Kazunori Kadota from the School of Pharmaceutical Sciences at Wakayama Medical University, has recently examined the effect of antisolvent addition rate and initial solute concentration on localized LLPS in a ternary water/ethanol/butylparaben system. Their insightful findings were made available online on 30 December 2025 and were published in Volume 444 of the Journal of Molecular Liquids on 15 February 2026.
In this study, the team measured the cloud point—the temperature below which a transparent solution undergoes either LLPS to form a turbid or cloudy emulsion or a solid-liquid phase transformation to form a precipitate—of their system under various conditions. They utilized the cloud point measurements to construct a non-equilibrium phase diagram. This diagram revealed that high antisolvent addition rates as well as high initial solute concentrations remarkably enhance the likelihood of LLPS.
Prof. Shirakawa highlights further implications of faster kinetics. “Rapid antisolvent addition induces local spinodal regions, triggering spontaneous phase separation. Moreover, our in-depth analysis of the diffusion behavior of antisolvent droplets in solution shows that high solute concentrations suppress mass transfer by slowing down antisolvent diffusion. This suppression leads to phase separation before local supersaturation is resolved, leading to solute-rich and solute-poor domains.”
Overall, the constructed non-equilibrium phase diagram offers practical insights for controlling LLPS, facilitating the design of novel, efficient and scalable crystallization processes that minimize LLPS and boost product quality in fine chemical manufacturing and other sectors.
“Our work visually connects the microscopic phenomenon of diffusion at the molecular level with the macroscopic phenomenon of droplet settling and floating, potentially paving the way for cheaper and better crystallization-based products in the market,” concludes Prof. Shirakawa.
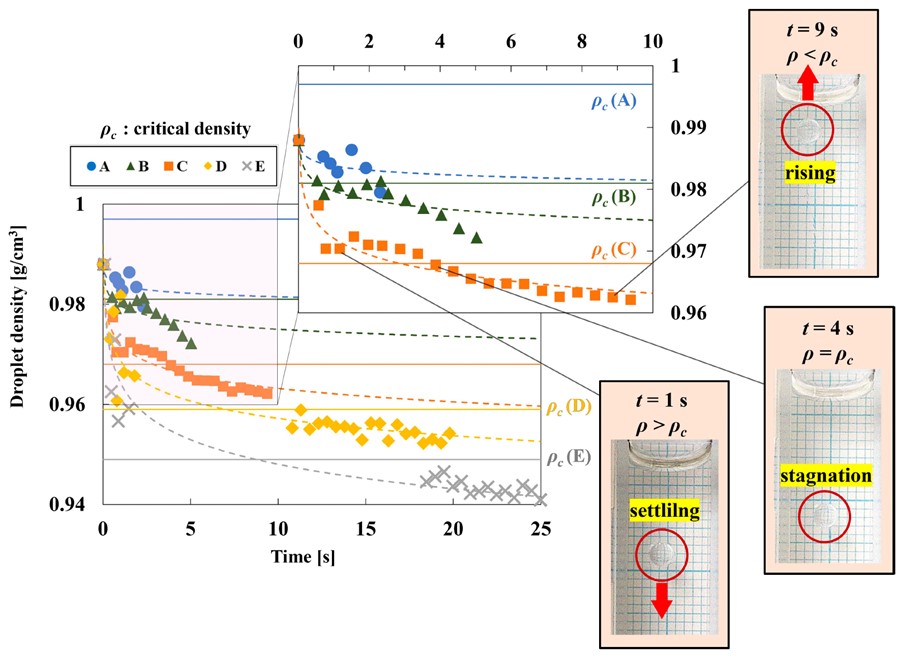
Characteristic behavior of droplets in solution during their diffusion
Researchers used cloud point measurements to construct a phase diagram. The solid lines represent the droplet density, while the dashed lines represent the solution density.
Credits: Professor Yoshiyuki Shirakawa from Doshisha University
Image license: ©Elsevier. Reproduced with permission from Journal of Molecular Liquids
Usage restrictions: This figure is used for press release purposes only.
Image link: https://www.sciencedirect.com/science/article/abs/pii/S0167732225024031
Reference
| Title of original paper | Non-equilibrium phase behavior of localized LLPS driven by solute concentration and antisolvent addition rate |
| Journal | Journal of Molecular Liquids |
| DOI | 10.1016/j.molliq.2025.129224 |
Profile

SHIRAKAWA Yoshiyuki
Dr. Yoshiyuki Shirakawa is a Professor at the Department of Chemical Engineering and Materials Science at Doshisha University, Japan. He received his Ph.D. and Master of Science degree from Niigata University in 1990 and 1993, respectively. His research interests include nanotechnology, functional solid-state chemistry, magnetism, superconductivity, strongly correlated systems, inorganic compounds and materials chemistry, and inorganic materials. He has authored over 200 research papers on these topics and received over 2,000 citations. Prof. Shirakawa is a member of The Society of Powder Technology, Japan; The Japan Institute of Metals; The Physical Society of Japan; The Japan Society of Applied Physics; and The Society of Chemical Engineers, Japan.
Professor
Faculty of Science and Engineering,
Department of Chemical Engineering and Materials Science
Funding information
This research was supported by JSPS KAKENHI (Grant No. JP24K08141), JST SPRING (Grant No. JPMJSP2129).
EurekAlert!
https://www.eurekalert.org/news-releases/1113754
Media contact
Organization for Research Initiatives & Development
Doshisha University
Kyotanabe, Kyoto 610-0394, JAPAN
CONTACT US
